Understanding the Power of Electrolysis
How does electrolysis work? At its core, electrolysis uses an electric current to split substances, like water, into their basic elements. In an electrolyzer, this process separates H₂O molecules into hydrogen gas (H₂) and oxygen gas (O₂).
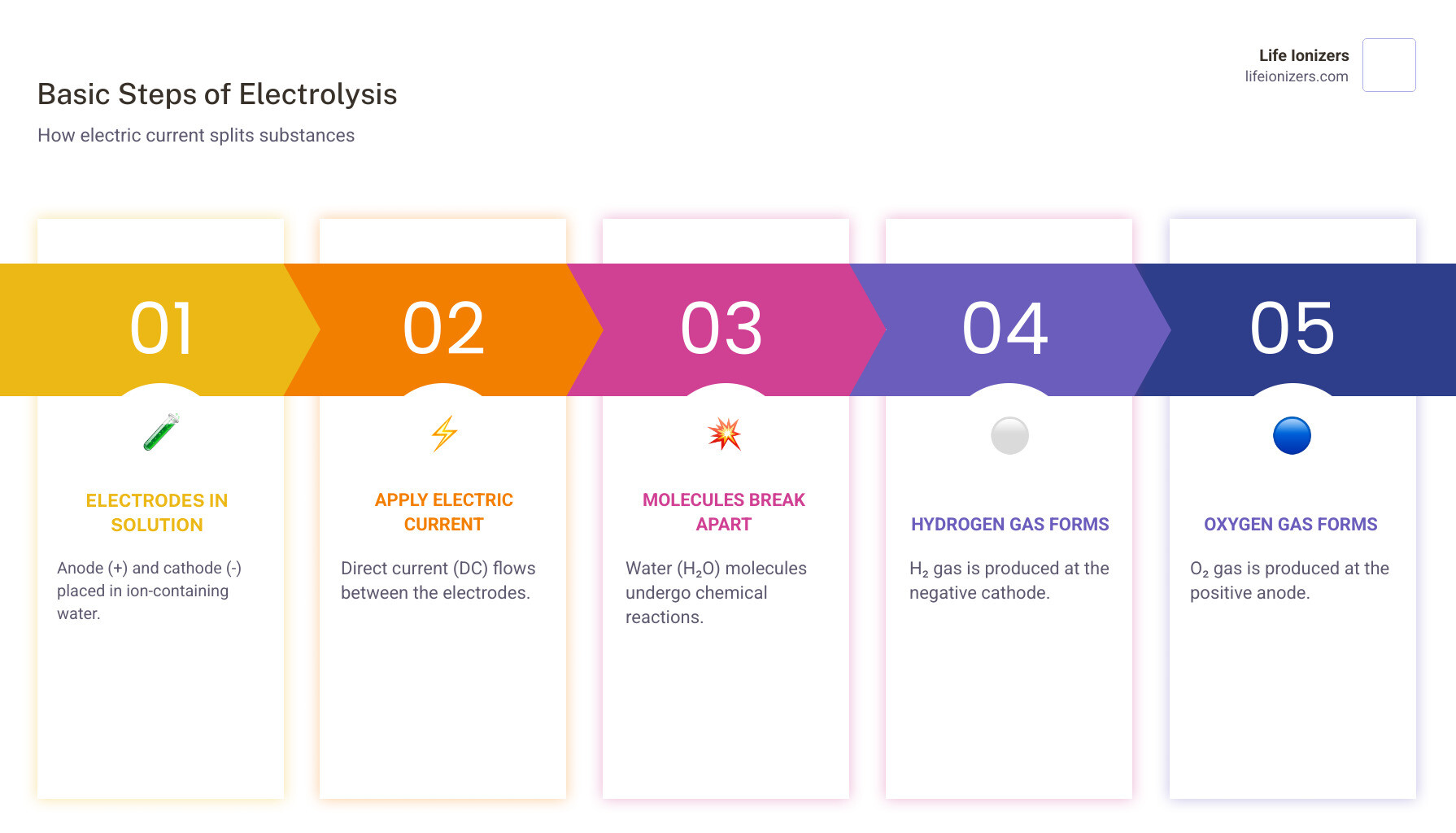
The basic process involves:
- Two electrodes (an anode and a cathode) placed in water containing ions
- A direct electric current flows between the electrodes
- Water molecules break apart at each electrode
- Hydrogen gas forms at the negative cathode
- Oxygen gas forms at the positive anode
This powerful process has transformed industries from aluminum production to green energy generation and is crucial for creating hydrogen-rich water for wellness.
Coined by scientist Michael Faraday in 1834, "electrolysis" literally means "electric splitting." What was once a lab curiosity is now essential technology, producing a growing share of the world's hydrogen as we seek carbon-free energy.
Understanding electrolysis is key to addressing two modern challenges: producing clean energy and creating pure, beneficial water. Whether you're interested in tap water quality or the antioxidant properties of hydrogen-rich water, electrolysis is the technology that makes it possible.
I'm Thai Cabados, and for over two decades I've worked with water electrolysis technology, from introducing the first alkaline hydrogen water systems to the United States to designing commercial-grade systems for physicians. How does electrolysis work in water ionizers is a question I've answered countless times, and I'm here to break it down for you.
The Fundamental Science: How Does Electrolysis Work?
In chemistry, electrolysis uses direct electric current (DC) to drive a chemical reaction that wouldn't happen on its own. It's like using energy to carefully pry apart a LEGO creation piece by piece, rather than letting it break apart spontaneously.
This process occurs in an electrolytic cell, which requires three key components:
- An electrolyte: A substance with free ions (like salt dissolved in water) that conducts electricity.
- Two electrodes: Electrical conductors, called the anode and cathode, that are placed in the electrolyte.
- A direct current (DC) power source: This supplies the energy to drive the reaction.
The DC power source creates an electrical field that causes ions in the electrolyte to move towards the electrodes. This movement leads to chemical reactions: oxidation (loss of electrons) at one electrode and reduction (gain of electrons) at the other. This fundamental process was defined by Michael Faraday in 1834 and builds on the work of pioneers like Alessandro Volta and Humphry Davy.
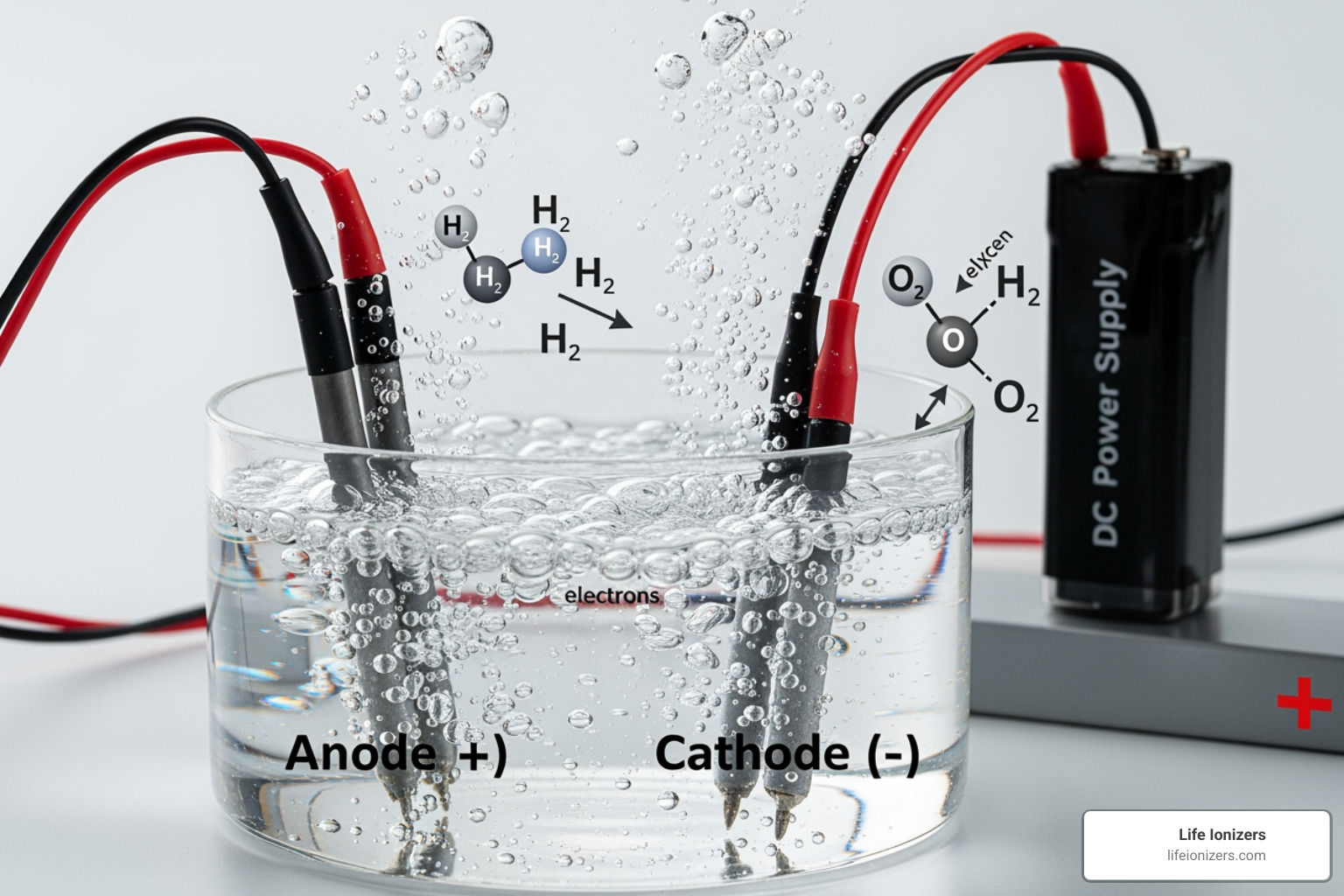
The Role of Electrodes and the Electrolyte
The electrodes and electrolyte are where the action happens.
- The Anode (Positive Electrode): This is where oxidation occurs. Negatively charged ions (anions) are attracted here, where they lose electrons. In water electrolysis, this is where oxygen gas is formed.
- The Cathode (Negative Electrode): This is where reduction takes place. Positively charged ions (cations) are drawn here to gain electrons. In water electrolysis, this is where hydrogen gas bubbles up.
The electrolyte is crucial because its free-moving ions carry the electric current through the solution, completing the circuit and allowing the reactions to occur.
How does electrolysis work in water?
The electrolysis of water splits H₂O molecules into hydrogen gas (H₂) and oxygen gas (O₂). This is the core process behind green energy initiatives and the creation of hydrogen-rich water.
Here’s a simplified breakdown of the reactions:
-
At the Anode (Oxidation): Water molecules lose electrons, producing oxygen gas.
- 2H₂O(l) → O₂(g) + 4H⁺(aq) + 4e⁻
-
At the Cathode (Reduction): Hydrogen ions gain electrons, forming hydrogen gas.
- 4H⁺(aq) + 4e⁻ → 2H₂(g)
The net result is that water splits into hydrogen and oxygen. These gases are collected separately, ensuring we get pure hydrogen—a versatile fuel and the key component of "Live Hydrogen Water™".
A Look Inside: Types of Electrolyzers and Their Function
Electrolyzers—the machines that perform water electrolysis—come in various types, much like cars. Some are built for reliability, others for quick response, and some for high-powered industrial use. The technology is highly scalable, from compact kitchen units like our Life Ionizers to massive facilities producing tons of hydrogen daily. Let's explore the three main types.

Alkaline Electrolyzers
As the most established technology (dating to 1927), alkaline electrolyzers are known for their reliability and affordability. They use a liquid alkaline electrolyte, typically potassium hydroxide (KOH), and operate at temperatures below 100°C.
- How it works: Hydroxide ions (OH⁻) travel through the liquid electrolyte, carrying the charge. A diaphragm separates the resulting hydrogen and oxygen gas.
- Pros: Proven, durable, and relatively low-cost.
- Cons: Slow to start up (around 50 minutes) and less responsive to fluctuating power, making them less ideal for pairing with intermittent renewables.
Proton Exchange Membrane (PEM) Electrolyzers
PEM electrolyzers are the sprinters, excelling at quick starts and rapid response. They are ideal for pairing with renewable energy and for applications requiring high purity, like water ionizers.
- How it works: A solid polymer membrane allows only protons (hydrogen ions) to pass through. Water splits at the anode, protons cross the membrane to the cathode, and they combine with electrons to form pure hydrogen gas.
- Pros: Fast startup (15 minutes), responsive to power changes, compact design, and high gas purity.
- Cons: Higher initial cost due to the use of precious metal catalysts like platinum and iridium.
Solid Oxide Electrolyzers
These are high-performance specialists, operating at extreme temperatures of 700°C to 800°C. They use superheated steam instead of liquid water.
- How it works: A solid ceramic electrolyte conducts oxygen ions (O²⁻). The high heat reduces the amount of electrical energy needed, boosting efficiency.
- Pros: Very high efficiency (around 80%), especially when using waste heat from industrial sources.
- Cons: Very long startup times (hours), and the extreme heat stresses materials, which can affect long-term durability. They are not suitable for small-scale or intermittent use.
Comparing Electrolyzer Technologies
Each technology has its place. Alkaline systems are workhorses for steady, large-scale production. PEM systems offer flexibility and purity, making them perfect for renewable energy integration and creating wellness water. Solid oxide systems deliver maximum efficiency in specific industrial settings with waste heat. Understanding these differences shows that how does electrolysis work depends entirely on the technology and the goal.
The Many Faces of Electrolysis: Key Applications
Electrolysis is a versatile technology working in more places than you might imagine, from powering a clean energy future to purifying the water in your home.
Hydrogen Production for a Green Future
One of the most exciting applications is creating green hydrogen—hydrogen produced using renewable energy like wind and solar. This process is completely clean, releasing no greenhouse gases. The resulting hydrogen is a versatile, carbon-free fuel that can:
- Fuel transportation (cars, buses, ships)
- Store renewable energy to stabilize power grids
- Decarbonize heavy industries like steel and chemical manufacturing
The U.S. Department of Energy's Hydrogen Energy Earthshot initiative aims to make clean hydrogen affordable, targeting a cost of $1 per kilogram within a decade. Electrolysis is the key technology making this green transition possible.
Major Industrial Uses
For over a century, electrolysis has been an industrial backbone.
- Aluminum Production: The Hall–Héroult process uses electrolysis to extract aluminum from ore, making this once-precious metal ubiquitous.
- Chlor-alkali Process: This process produces chlorine, sodium hydroxide, and hydrogen—essential building blocks for thousands of products like PVC, paper, and detergents.
- Metal Processing: Electroplating (coating metals), electrowinning (extracting metals), and electrorefining (purifying metals) are all critical industrial applications.
How does electrolysis work for other applications?
Electrolysis also appears in more personal applications:
- Permanent Hair Removal: The FDA-approved method uses an electrical current to destroy hair follicles.
- Rust Removal: A gentle method used by hobbyists to restore metal tools and antiques by reversing oxidation.
- Water Purification: This is the technology at the heart of Life Ionizers. Our advanced ionizers use electrolysis to create "Live Hydrogen Water™," rich in antioxidant molecular hydrogen. The process separates beneficial minerals from unwanted contaminants, and a variation called electrodeionization is used to create ultra-pure water for labs.
From the fuel in future vehicles to the water in your glass, electrolysis is quietly making modern life cleaner and better.
The Future of Electrolysis: Challenges and Innovations
The future of electrolysis is focused on making the technology more efficient, affordable, and durable. The main question for researchers is no longer if it works, but how does electrolysis work even better?
Key challenges include:
- Overpotential: The extra energy required above the theoretical minimum, which increases electricity costs.
- Catalyst Cost: The most effective catalysts often use expensive and rare precious metals like platinum.
- Durability: Electrodes can degrade over time, especially under harsh conditions, reducing efficiency.
Researchers are particularly focused on improving the slow and energy-intensive "Oxygen Evolution Reaction" at the anode, which could open up significant efficiency gains.
Advantages and Disadvantages of Electrolysis
Like any technology, electrolysis has trade-offs.
Advantages:
- Zero Emissions: When powered by renewables, it produces no greenhouse gases.
- Energy Storage: It can store excess renewable energy as hydrogen.
- High Purity: It creates very pure products, from industrial hydrogen to the wellness water from Life Ionizers.
- Flexibility: PEM systems can respond quickly to fluctuating power from sources like wind and solar.
Disadvantages:
- High Energy Use: The process is electricity-intensive. If the power comes from fossil fuels, it's not truly "green."
- Cost: Electricity and expensive catalysts contribute to high operational costs.
- Infrastructure: Building out a large-scale hydrogen storage and distribution network is a major undertaking.
Overcoming Problems: Cost and Efficiency
The good news is that innovation is accelerating. To meet the U.S. Department of Energy's goal of reducing clean hydrogen cost by 80% (to $1/kg in a decade), scientists are developing:
- Cheaper Catalysts: Creating new catalysts from abundant materials to replace precious metals.
- Improved Durability: Lowering operating temperatures for high-temperature systems to reduce material stress.
- Novel Approaches: Exploring methods like carbon-assisted water electrolysis (CAWE) to reduce electricity needs and direct seawater electrolysis to conserve freshwater.
Each breakthrough brings us closer to a future where clean hydrogen is a mainstream reality and advanced electrolysis continues to create the purest, healthiest water.
Frequently Asked Questions about Electrolysis
What is the difference between electrolysis and a galvanic (voltaic) cell?
This question gets to the heart of energy flow. The key difference is:
- Electrolysis consumes energy. It uses electrical energy to force a chemical reaction to happen that wouldn't occur on its own. In an electrolytic cell, the anode is positive and the cathode is negative.
- A galvanic cell (a battery) produces energy. It generates electricity from a spontaneous chemical reaction that happens naturally. In a galvanic cell, the polarity is reversed: the anode is negative and the cathode is positive.
In short, one uses power, and the other creates it.
Can you perform electrolysis on pure water?
While technically possible, performing electrolysis on pure water is extremely inefficient. Pure water is a very poor conductor of electricity because it contains almost no free ions to carry a current.
To make the process work effectively, an electrolyte (a small amount of a salt, acid, or base) must be added. The electrolyte dissolves into ions, which dramatically increases the water's conductivity and allows the electric current to flow smoothly. The minerals naturally present in tap water act as electrolytes, which is why our Life Ionizers are designed to work with your water's specific mineral content to produce the best results.
What determines the products of electrolysis?
The final products of electrolysis depend on several factors. Understanding how does electrolysis work means considering the entire system. Key factors include:
- The Substance: The primary material being electrolyzed (e.g., water vs. molten salt) determines the possible products.
- The Electrodes: Electrodes can be inert (like platinum), simply facilitating the reaction, or reactive, becoming part of the chemical change.
- Ion Concentration: The concentration of different ions in the electrolyte can favor one reaction over another.
- Overpotential: The extra voltage needed to drive a reaction can influence which of several competing reactions occurs.
- Temperature: Especially in high-temperature systems, this dramatically affects reaction pathways and efficiency.
By controlling these variables, we can precisely direct electrolysis to create desired products, whether it's pure aluminum for industry or molecular hydrogen for your health with a Life Ionizer.
Conclusion
From Faraday's early experiments to today's cutting-edge green hydrogen facilities, we've explored how electrolysis transforms our world. This neat process—using electricity to drive chemical change—has become far more than a laboratory curiosity. It's reshaping industries, powering our transition to clean energy, and yes, even improving the water we drink every day.
How does electrolysis work isn't just a technical question. It's the key to understanding technologies that will define our future, from carbon-free transportation to sustainable manufacturing. When you grasp that simple principle of electrons flowing through water, splitting molecules into their elements, you're seeing the foundation of solutions to some of our biggest challenges.
For those of us at Life Ionizers, this technology hits close to home—literally. Every day, we apply these same advanced electrolysis principles to create something personal and immediate: water that supports your health and vitality. Our XL Matrix GRID Technology uses precision-engineered platinum-coated titanium plates to produce "Live Hydrogen Water™" right in your kitchen, bringing the benefits of molecular hydrogen directly to you and your family.
After two decades in this field, I still find it remarkable that the same scientific principles powering massive industrial electrolyzers are also working quietly in homes across America, including right here in Vista, CA. It's a powerful reminder that breakthrough technology doesn't have to be distant or abstract—it can be as close as your kitchen counter.
As researchers continue pushing the boundaries of efficiency and cost-effectiveness, and as our nation invests in hydrogen infrastructure and renewable energy, electrolysis will only grow more important. Whether it's producing green hydrogen for fuel cells or creating antioxidant-rich water for your morning glass, this technology is here to stay.
If you're curious about how our electrolysis technology compares to other systems and what it might mean for your health, I invite you to dig deeper: Compare molecular hydrogen water machines. The future of water wellness is already here, and it's powered by the same principles that are helping power our planet's sustainable future.

